Cohesion and Surface Tension
The cohesive forces between molecules in a liquid are shared with all neighboring molecules. Those on the surface have no neighboring molecules above and, thus, exhibit stronger attractive forces upon their nearest neighbors on and below the surface. Surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water molecules.Surface tension at a molecular level
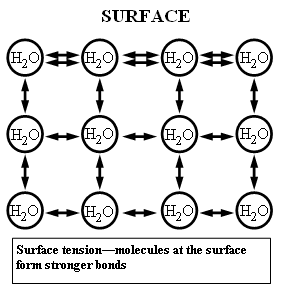 Water
molecules want to cling to each other. At the surface, however, there
are fewer water molecules to cling to since there is air above (thus, no
water molecules). This results in a stronger bond between those
molecules that actually do come in contact with one another, and a layer
of strongly bonded water (see diagram). This surface layer (held
together by surface tension) creates a considerable barrier between the
atmosphere and the water. In fact, other than mercury, water has the
greatest surface tension of any liquid. (Source: Lakes of Missouri)
Water
molecules want to cling to each other. At the surface, however, there
are fewer water molecules to cling to since there is air above (thus, no
water molecules). This results in a stronger bond between those
molecules that actually do come in contact with one another, and a layer
of strongly bonded water (see diagram). This surface layer (held
together by surface tension) creates a considerable barrier between the
atmosphere and the water. In fact, other than mercury, water has the
greatest surface tension of any liquid. (Source: Lakes of Missouri)Within a body of a liquid, a molecule will not experience a net force because the forces by the neighboring molecules all cancel out (diagram). However for a molecule on the surface of the liquid, there will be a net inward force since there will be no attractive force acting from above. This inward net force causes the molecules on the surface to contract and to resist being stretched or broken. Thus the surface is under tension, which is probably where the name "surface tension" came from. (Source: Woodrow Wilson Foundation).
Due to the surface tension, small objects will "float" on the surface of a fluid, as long as the object cannot break through and separate the top layer of water molecules. When an object is on the surface of the fluid, the surface under tension will behave like an elastic membrane
No comments:
Post a Comment