Radioactivity
1. Radioactivity
is the spontaneous and random emission of radioactive rays from
unstable radioactive materials after which they become more stable.
2. The
process is said to be spontaneous because it is not influenced by any
physical factors such as temperature, pressure, time, etc.
3. A nucleus is unstable if it is too big. All nuclei with z > 83 or A> 209 are unstable.
4. The emission of radioactive rays is random means that
- Emission occurs at irregular intervals.
- Emission does not occur at the same means.
5. There are three different types of radioactive emissions.
- Alpha particle- a
- Beta particle- B
- Gamma ray-r
6. Table below shows the characteristics of alpha particle, beta particle, and gamma particle.
|
Characteristic
|
Alpha particle
|
Beta particle
|
Gamma ray
|
|
Nature
|
Positively charged helium nucleus, He
|
Negatively charged electron, e
|
Neutral electromagnet ray
|
|
In an electric field
|
Bends to the negative plate
|
Bends to the positive plate
|
Does not bend, showing that it is neutral.
|
|
In magnetic field
|
Bends a little showing that it has a big mass. Direction of the bend indicates that it is positively charged.
|
Bends a lot showing that it has a small mass. Direction of the bend indicates that it is negatively charged.
|
Does not bend showing that it is neutral.
|
|
Ionising power
|
Strongest
|
Intermediate
|
Weakest
|
|
Penetrating power
|
low
|
Intermediate
|
High
|
|
Stopped by
|
A thin sheet of paper
|
A few millimeters of aluminium
|
A few centimeters of lead or concrete
|
|
Range in air
|
A few centimeters
|
A few metres
|
A few hundred metres
|
|
Speed
|
1/20 X the speed of light, c
|
3%-99% of the speed of light, c
|
The speed of light,c
|
Nuclear Reactor
2. The energy freed from the fusion of nuclear fuel heats the water in the surrounding.
3. Consequently, this produces steam which drives the turbines. The turbines then drive the electrical generators.
4. The table below summarises the main functions of each components.
Component
|
Function / Explanation
|
Graphite Moderator
|
Fast moving neutrons are slowed down by collisions with
nuclei in the moderator so that they can cause further fissions. In some
nuclear power plant, the moderator is water.
|
Uranium rod (Fuel)
|
Fission reactions take place in the uranium rod to create
nuclear energy. The uranium used is often ‘enriched’ by increasing the
proportion of the isotope uranium-235 above the natural value of 0.7% to 3%.
|
Control Rod
|
The rate of the fission reaction is controlled by
inserting or withdrawing these rods. The nuclei in the rods absorb neutrons
without undergoing any reaction. Sometimes the rod is made of cadmium.
|
Coolant
|
To take away heat from the nuclear reactor.
Substances with high specific heat capacity such as ‘heavy’ water and carbon
dioxide are used.
|
Thick Concrete Wall
|
To avoid the run off of harmful radiations.
|
Steam generator
|
Water in the generator is heated and changed into
steam. The steam then drives the turbines.
|
Turbines
|
To revolve the dynamo in the electrical generator to
generate electricity
|
5. Nuclear reactors are used in the production of:
a) High-intensity neutron beams for research
b) Artificial Radioactive Isotopes for medical research
c) Fissionable transuranic elements such as plutonium from uranium-238
Reasons for the use of Nuclear Energy
1. Production of nuclear energy from nuclear fuels involves a decreased cost. A small amount of nuclear fuel can provide a large amount of energy.
2. Nuclear reactors are relatively safe especially with the sophisticated technology constantly developed and improved.
3. The decreasing supply of fossil fuels make it essential for the use of alternative sources of energy.
4. The use of nuclear energy does not release greenhouse gases such as carbon dioxide.
Reasons against the use of Nuclear Energy
1. Radioactive residues from nuclear stations have quite a long half-lives.
2. There is a chance of leakage in the radioactive waste containers placed underground or underwater.
3. High cost of constructing a nuclear power station.
4. Accidents could happen due to human error no matter how sophisticated the technology is and this should be put into consideration
Precautionary Steps In Handling Radioactive Substance
Weak radioactive substances could be handled by using tweezers.
Radioactive wastes must be disposed off by using suitable and safe methods. Rooms, buildings, containers and radioactive storage places must be labelled with the sign for radioactive substance. Radioactive substances are contained in thick lead containers.
Protective suits and gears such as gloves and eye glasses made of lead are used at all times when handling radioactive substances. These shields protect the workers from harmful radiations.
Workers handling radioactive substances must wear special badges which detect the amount of radiation they are exposed to. Food and drinks are not allowed in places where radioactive substances are handled.
Low level radioactive wastes
Sources: Hospitals, nuclear power stations, industries, research laboratories.
Examples: Contaminated equipments, shoes, biohazard suit, clothing, wrappers, air filters, gloves, etc.
Half-life: Short
Radioactivity level: low
Management: Solid wastes are stored
Intermediate level radioactive wastes
Sources: Nuclear power stations, industries, research laboratories
Examples: Component in nuclear reactors, chemical sediments
Half life: long
Radioactivity level: High
Management: Radioactive wastes are placed in concrete block and then buried underground
High level radioactive wastes
Sources: Nuclear power stations
Examples: Fuel rods used in nuclear power stations
Half life: Long
Radioactivity level: High
Management: Fuel rods are submerged in a pool of water to cool them down. The rods are then stored in a steel container which are buried underground at a depth of between 500m and 600m.
importance of proper management of radioactive substances
Negative effects of radioactive substances
Radioactive substances emit radiations that are harmful to living things. This is due to the ionisation and penetrating properties of these radiations.
As the radiations pass through living cells, they ionise the neighbouring atoms or molecules. The reactive ions that are produced will
i. Interfere with the chemical processes in the cell.
ii. Induce mutations in the genetic structure of the cell.
At the same time, the radiations might kill the cell in body tissues. If there are far too many cells that were destroyed, the organism may die.
The amount of damage inflicted to humans depends on the types of radiation, dosage and exposure period, methods of insertion into the body and location of exposure.
i. Types of radiation - Alpha particles outside the body are harmless because they can be stopped by the human skin.
ii. Dosage and exposure - Exposure to high dosage of radiation in a short period of time results in immediate symptoms such as vomitting, increase in body temperature, blood composition change and many more.
iii. Methods of insertion into the body - The internal part of human body can be damaged by alpha particle that were ingested through food or inhaled through air, this is due to the high ionising effect of Alpha particles.
iv. Cells that are actively dividing are more vulnerable to radiations. Skin cells in general can withstand higher dosage of radiation compared to the other internal organ.
The harmful effects of radiation on humans can be divided into two categories which can be categorised as Somatic effect or Genetic effect.
i. Somatic effect: includes damage to all parts of the body except the reproductive organs. Symptoms include: fatigue, vomiting, hair loss, infertility in male, severe skin burn and leukemia or cataracts (which may arise after a long period of time).
ii. Genetic effect: includes damage to reproductive cells. Genetic defect can be passed down to the next generations. Examples of genetic defects include Down Syndrome, Klinefelter Syndrome, Turner Syndrome.
Nuclear Fusion
This process is accompanied by the release of a huge amount of energy.
For example
Deuterium + Tritium = Helium + neutron + (Energy)
(2H1) + (3H1) = (4He2) + (1n0) + Energy
It is far more difficult to achieve fusion than fission due to the nature of the hydrogen nuclei that repel each other. In order to let this happen, the nuclei should be heated up to 10^8 K or more so that the nuclei will have enough kinetic energy to overcome the electrical repulsion between the nuclei.
The Sun acquires its energy from the fusion of hydrogen nuclei.
- Deuterium clashes with tritium to form a helium nucleus at a high temperature. This is accompanied by the release of a neutron and mass defect. The mass defect produces a massive amount of energy.
A hydrogen bomb also uses the principle of nuclear fusion for its design.
Nuclear Fission
Nuclear fission seldom occurs spontaneously. Usually it occurs when the heavy nucleus is bombarded by a neutron.
Induced fission occurs when reaction caused by neutrons absorption.
Spontaneous fission occurs when nuclei undergo fission without initial neutron absorption.
The reaction causes large mass defect which appears mostly as kinetic energy of the fission fragments. They fly apart at great speed and colliding with the atoms in the vicinity and increasing their average kinetic energy. This causes them to have higher temperature and give out heat. Thus, nuclear fission is one of the source of heat energy.
Nuclear Energy : atomic mass unit and nuclear energy
Atomic mass unit (a.m.u)
The atomic mass unit (a.m.u) is used to measure the masses of atomic particles.
1 a.m.u is defined as (mass of carbon-12 atom / 12)
i.e. mass of carbon-12 atom divided by 12.
It is known that the mass of one carbon-12 atom is 1.99 X 10^ - 26 kg.
Therefore,
1 a.m.u = (1.99 X 10^ - 26 kg / 12)
= 1.66 X 10 ^ - 27 kg
The value is very close to the mass of one proton or neutron.
The atomic mass unit (a.m.u) is often used in nuclear physics as it is easier unit to represent masses of minute particles.
Nuclear Energy
In a radioactive disintegration, a process where one element changes into another is called transmutation.
The mass of daughter particles and other particles produced is less than
that of the different particle. This difference in mas is called mass
defect or mass loss.
Mass defect = mass of parent particle - Total mass of daughter particles and other particles produced.
The mass loss is converted into energy.
According to Einstein's principle of Mass-Energy Conservation, the
change of energy is linked to the change of mass by the equation;
E = mc^2
m = mass change, kg
c = speed of light, ms-1
E = energy changed, J
Applications of Radioisotopes in Different Fields
i. A stable nucleus is bombarded by high speed alpha particles, neutrons or protons to produce artificial radioisotopes.
ii. The bombarding particles are trapped in the nucleus creating a radioactive isotopes.
Among the properties of radioisotopes are:
i. Emits radioactive radiation.
ii. Radioactive radiations can kill cells.
iii. Radioactive radiations have different penetrating ability with materials of different thickness and densities.
iv. Radioactive radiations can cause cell mutation.
v. Radioactive radiations can ionise molecules.
vi. Its activity decreases with time.
vii. Radioisotopes have the same chemical properties as non-radioactive isotopes of the same element.
Applications of radioisotopes in Medicine
1. To diagnose of thyroid disease using iodine-123.
2. To treat an overactive thyroid gland and certain kinds of thyroid cancer by using sodium iodide labelled with radioactive iodine.
3. To detect position of blood clots or thrombosis using Sodium-24 injected in the bloodstream.
4. To detect and treat brain tumor using phosphorus-32
5. To study the circulation of iron in the blood using iron-59
6. To sterilise medical equipments and to destroy cancer cells in radiotherapy radioisotope cobalt-60 is used.
Applications of radioisotopes in Industries
1. The thickness of paper, plastics, clothes and metal sheets need to be standardised and this is done by placing a raioactive source at one side of the material and a detector on the other side.
2. For sheets of metal, gamma ray is used. For plastics, clothes and paper, beta particles are used.
3. The detector will register a higher count if the material is too thin and lower register if too thick. The computer will make adjustments according to the thickness of the material.
4. This mechanism is also used to ensure that containers such as cans and food packages are filled to the specified amount.
5. Radioisotope is added to engine oil so that its level of wear and tear can be determined.
6. In order to kill germs that cause food to spoil quickly, gamma rays are used.
7. If exposed to gamma ray, latex becomes harder without the need for adding sulphur.
Applications of radioisotopes in Agriculture
1. Pests can be killed using radioactive rays esp using gamma rays.
2. To stop pests from reproducing, induced mutation by using gamma rays can be employed. But this has the probability of producing GMO and resistant pests.
3. To be used as tracers in the effectiveness of fertilisers using nitrogen-15 and phosphorus -32.
4. To induce genetic mutation in a plant in order to produce a better strain which has higher resistance against pest and diseases.
Applications of radioisotopes in Archaeology
1. To determine the age of artifacts, the carbon dating method is used.
Consider this paragraph:
Cosmic radiations from outer space displaced neutrons from nuclei in the Earth's outer atmosphere. These neutrons then collide with nitrogen nuclei to produce carbon-14. Living organisms like plants and animals absorb and give out carbon-14 when they are alive. The half-life of carbon-14 is about 5730 years. So there is negligible disintegration over the lifetime of most organisms. However when they die, no more absorption of carbon-14 occurs. The C-14 taken starts to decay into N-14 by beta emission. The percentage of carbon 14 in dead plant decreases as the carbon 14 disintegrates. After 5730 years, the percentage of carbon 14 falls to 50 percent of its initial value. The activity of atoms is proportional to the number of undecayed atoms. By comparing the activity of the dead sample of the same mass of the living sample, its age can be estimated.
2. To measure geological time.
During the formation of rocks, some radioisotopes such as uranium-238 are trapped. As the decay continues, the proportion of uranium-238 decreases slowly resulting in the equally slow growth of its product lead-206. An estimate of the age of the rock can be inferred from the relative proportions of lead and uranium in the rock.
Radioisotopes

Examples of half lives of some common isotopes
Determining the Half-life
Nuclei in a radioactive sample disintegrate at random.
Each nucleus has the equal chance of being decayed. Which means that at any time, any nuclei can decay / disintegrate.
Activity = the average number of decay or disintegrations per unit time in a radioactive sample.
During the decay of a radioactive sample, the number of atoms which have disintegrated increases, while the number of atoms which have not disintegrated decreases. It has to be remembered that the total number of atoms remain constant during this process.
The rate of decay lessens as the number of intact atoms that remain decreases and thus activity decreases with time as the number of undecayed atoms decreases.
It has to be noted that different radioactive elements decay at different rates.
Point: Half-life, t½ , of a radioactive isotope is the time taken for the activity of atoms of that isotope to fall to half of its original value.
also
Half-life (t½) can also be stated as the time taken for the number of radioactive atoms to decrease to half of its original number.
Consider this:
i. If N is the number of original atoms in a radioactive sample.
ii. After one half-life has lapsed, half (1/2)N atoms remain and half (1/2)N atoms have disintegrated.
iii. After two half-lives, (1/2)X(1/2)N = (1/2)^2 N = (1/4) N atoms remain and (3/4)N atoms have disintegrated.
*Remember: N - (1/4) N = (3/4) N
iv. This decay process continues until a stable atom is produced.
v. Say x = number of half lives
N = original number of atoms
Nx = number of atoms remaining after x half-lives, then we can say that
Nx = (1/2)^x N
(Remember the symbol ^ means to the power of)
Also, it is worth to consider these formulas:
Source: wikipedia.com
-->
Radioisotopes are isotopes with radioactive properties.
Isotopes are elements whose atoms have the same number of proton but different number of neutrons.
Isotopes have different nucleon numbers but the same proton number.
Radioisotopes is isotopes with unstable nuclei.
Synthetic isotopes are produced by unstable nuclei that decay.
There are many uses of radioisotopes in the field of medicine, agriculture, industry and research.
Radioisotopes as used as tracers in scientific research, medical diagnosis and industry.
Half Life
Half-life
Concept of Half-life
The reactivity or activity of a radioactive material is the rate of decay of the material.
The rate of decay is the same as the number of atoms which decay or are emmited every second.
The rate of decay of a radioactive materials depends on the number of
atoms that have not yet undergone decay. Thus, the reactivity of a
radioactive material will decrease with time.
The half-life (t½) of a radioactive element is the time taken for half the number of atoms in a sample of radioactive atoms to decay.
After one half-life, the activity and the number of atoms remaining of any radioactive substance are halved.
Decay curve.
The half-life of the same radioactive element is the same but the half-lives of different radioactive elements are different.
The value of half-life is not influenced by factors such as temperatures, pressure and etc.

(Source: bbc.co.uk)
Examples of half lives of some common isotopes
Radioisotope
|
Half-life
|
Uranium-238
|
5000 million years
|
Uranium-235
|
700 million years
|
Plutonium-239
|
24 000 years
|
Carbon-14
|
5700 years
|
Calcium-137
|
30 years
|
Cobalt-60
|
5 years
|
Determining the Half-life
Nuclei in a radioactive sample disintegrate at random.
Each nucleus has the equal chance of being decayed. Which means that at any time, any nuclei can decay / disintegrate.
Activity = the average number of decay or disintegrations per unit time in a radioactive sample.
During the decay of a radioactive sample, the number of atoms which have disintegrated increases, while the number of atoms which have not disintegrated decreases. It has to be remembered that the total number of atoms remain constant during this process.
The rate of decay lessens as the number of intact atoms that remain decreases and thus activity decreases with time as the number of undecayed atoms decreases.
It has to be noted that different radioactive elements decay at different rates.
Point: Half-life, t½ , of a radioactive isotope is the time taken for the activity of atoms of that isotope to fall to half of its original value.
also
Half-life (t½) can also be stated as the time taken for the number of radioactive atoms to decrease to half of its original number.
Consider this:
i. If N is the number of original atoms in a radioactive sample.
ii. After one half-life has lapsed, half (1/2)N atoms remain and half (1/2)N atoms have disintegrated.
iii. After two half-lives, (1/2)X(1/2)N = (1/2)^2 N = (1/4) N atoms remain and (3/4)N atoms have disintegrated.
*Remember: N - (1/4) N = (3/4) N
iv. This decay process continues until a stable atom is produced.
v. Say x = number of half lives
N = original number of atoms
Nx = number of atoms remaining after x half-lives, then we can say that
Nx = (1/2)^x N
(Remember the symbol ^ means to the power of)
Also, it is worth to consider these formulas:
An exponential decay process can be described by any of the following three equivalent formulas:
where
- N0 is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc.),
- N(t) is the quantity that still remains and has not yet decayed after a time t,
- t1/2 is the half-life of the decaying quantity,
- τ is a positive number called the mean lifetime of the decaying quantity,
- λ is a positive number called the decay constant of the decaying quantity.
The three parameters 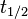 ,
,  , and λ are all directly related in the following way:
, and λ are all directly related in the following way:
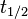 ,
,  , and λ are all directly related in the following way:
, and λ are all directly related in the following way:Source: wikipedia.com
Usage of Half-life
Half-life in Archeology
Carbon-14 has a half-life of 5600 years.
Humus, animals and plants absorb carbon-14 through carbon dioxide gas in
the atmosphere. A small amount in CO2 exists as carbon-14.
Living animals and vegetable have a constant amount of Carbon-14 because the c-14 decayed will always replaced.
However or dead beings the amount of C-14 in it will decrease because
new C-14 will not be absorbed causing its reactivity to decrease.
When an antique or human skill are found, their age can be determined by
Measuring the reactivity of C-14 in it.
Determine the ratio of decay carbon-14 against intact carbon-14.
Industries:
Radioisotopes can be used as tracers, in order for it to be feasible,
the radioisotopes used must have long enough half-lives. For example:
the leaks in undergound pipes carrying oil can be detected by injecting
radioactive tracer into the flow. Afterwards a GM tube can be utilised
to sense the leakage from the surface above the pipe.
Medicine:
To be useful in the medical field, the radioisotope must have a short
half-life. This is to prevent over exposure to radiation for an
unnecessarily long period of time.
The isotope Iron, 59Fe with a half life of 45 days is used in testing for iron in blood plasma.
Iodine, 131 I with a half life of 8 days can be used in thyroid tests and treatments.
Radioactive Detectors
Radioactive Detectors
Geiger-Muller Tube
- The Geiger-Muller tube is an effective radioactive detector. It can trace alpha particles, beta particles and gamma rays.
- The outer part of the G-M tube is made of aluminium which acts as the cathode.
- The middle part of the G-M tube is a metal wire which acts as the anode.
- The G-M tube is filled with argon gas at low pressure.
- Initially, the G-M tube must be connected to a high voltage before being used.
- This high voltage causes some ionization of argon gas.
Cloud Chamber
- The cloud chamber is made by using a transparent plastic box. The space in it is divided into two parts by a metal.
- The lower part is filled with solid carbon dioxide. Sponge is used to push the solid carbon dioxide towards the metal plate.
- The upper part is filled with molecules of alcohol vapour released from the felt which is initially soaked in alcohol.
- When the alcohol vapour diffuses downwards, it will become colder. Thus, a supersaturated condition will be produced in the space in the lower part of the chamber.
- When the radioactive rays enter the upper part, the ionization of air will occur. Saturated alcohol vapour will move above the ions. Droplets of liquid alcohol on the ions will cause the formation of misty tracks.
- Steps to ensure clear tracks:
- The transparent Perspex cover is rubbed with a soft cloth to produce charges which will remove all ions in the chamber before any radioactive rays enter.
- The cloud chamber must be placed horizontally to ensure smooth flow of particles in it.
- If light is used, it must shine on the area superated with vapour and not on the black base of the chamber in order to avoid heating it.
- Normally, the tracks produced are not uniform. This shows that the radioactive rays are produced randomly.
- There are three types of tracks as shown in Table below.
Types of radioactive rays
|
Explanation
|
|
The alpha tracks are thick and straight. This shows that alpha particles have the strongest ionizing power and the biggest mass.
|
|
The beta tracks are thin and curvy. This shows that beta particles have low ionizing power and small mass.
|
|
Their tracks are short, curvy and spiky from the middle. It shows that it has the lowest ionizing power.
|
- The number of radioactive tracks produced will decrease after a while. This is because after some time, the condensation of alcohol vapour on the radioactive source will block the emission of radioactive rays.
Spark counter
The wire gauze and thin wire are connected to a voltage of more than 2000 V.
The voltage is increased slowly until sparks are produced in between.
The sparks are formed due to ionisation of the air.
The voltage is then decreased until no sparks are formed.
The radioactive source is brought close to the wire gauze.
The
radioactive rays will ionize the air molecules between the wire gauze
and thin wire. Positively charged ions will be attracted to the
negatively charged gauze and the negatively charged ions will be
attracted to the positively charged thin ions.
Secondary ionization will occur due to the collision between the ions and the air molecules.
Therefore, sparks are formed.
The number of sparks measured the intensity of radioactive rays from its source randomly.
The spark counter can only trace alpha particles which have high ionizing power.
Electroscope
When charged plate of the electroscope is exposed to the source of alpha particles, the gold leaf will collapse slowly.
This is due to the ions and electron are produced by the alpha particles which will neutralize the charge in the electroscope.
The rate of collapse of the gold leaf indicates the strength of the radioactive source.
Photographic Plate
All types of radioactive rays will darken the photo film. The effect is like sunlight acting on it.
The ionization effect by the radioactive rays will decompose silver bromide crystals on the film.
Films which are exposed to sunlight will show white lines representing radioactive tracks.
Films are kept in the badges worn by workers as a tracer device of radioactive rays.
The
main disadvantage of using a film as a radioactive tracer is that it
needs to be processed in order to prove the presence of radioactive
rays.
Nucleus of an Atom
-->
The Composition of the Nucleus
1. Matter is made up of very small particles called atoms.
2. Each atom has a very small and very dense core known as the nucleus.
3. Most of the mass of the atom is contained in the nucleus.
4. The electrons move in orbits around the nucleus.
5. The diameter of the nucleus is about 100 000 times smaller than the diameter of the atom.
6. This means that there are lots of empty spaces within an atom.
7. The subatomic particles in a nucleus are called nucleons.
8. The two types of nucleons are protons and neutrons.
9. The proton is a positively charged particle. It carries a charge of +e, where e is equal to 1.6 × 10-19 C.
10. The neutron carries no charge. The neutrons has approximately the same mass as the proton.
11. The number of protons in the nucleus of an atom is known as the proton number, Z.
12. The total number of protons and neutrons in the nucleus of an atom is known as nucleon number, A or mass number.
13. Then number of neutrons, N = A – Z
Nuclide Notation
1. A nuclide is a type of atom with a particular nucleon number. This term is also used for a type of nucleus.
2. The nuclide notation of an atom gives the symbol of the elements, the proton number and the nucleon number of the atom.
Isotopes
1. Isotopes are atoms of the same elements with the same numbers of protons but different number of neutrons.
2. isotopes have the same proton number but different nucleon numbers.
3. All isotopes of an element have the same chemical properties because their electrons are arranged in exactly the same way.
4. Their physical properties such as densities, boiling points and melting points are different.
5. Some elements in nature such as oxygen,carbon, and bromine consist of a mixture of isotopes.
6. Some isotopes of an element are stable while some are unstable. The unstable isotopes or radioisotopes.
7. Radioisotopes
will undergo spontaneous decay to emit radioactive rays such as alpha,
beta and gamma rays. After radioactive decay, the proton number and
nucleon number of the radioisotope may be changed.




No comments:
Post a Comment