 ch301.cm.utexas.edu
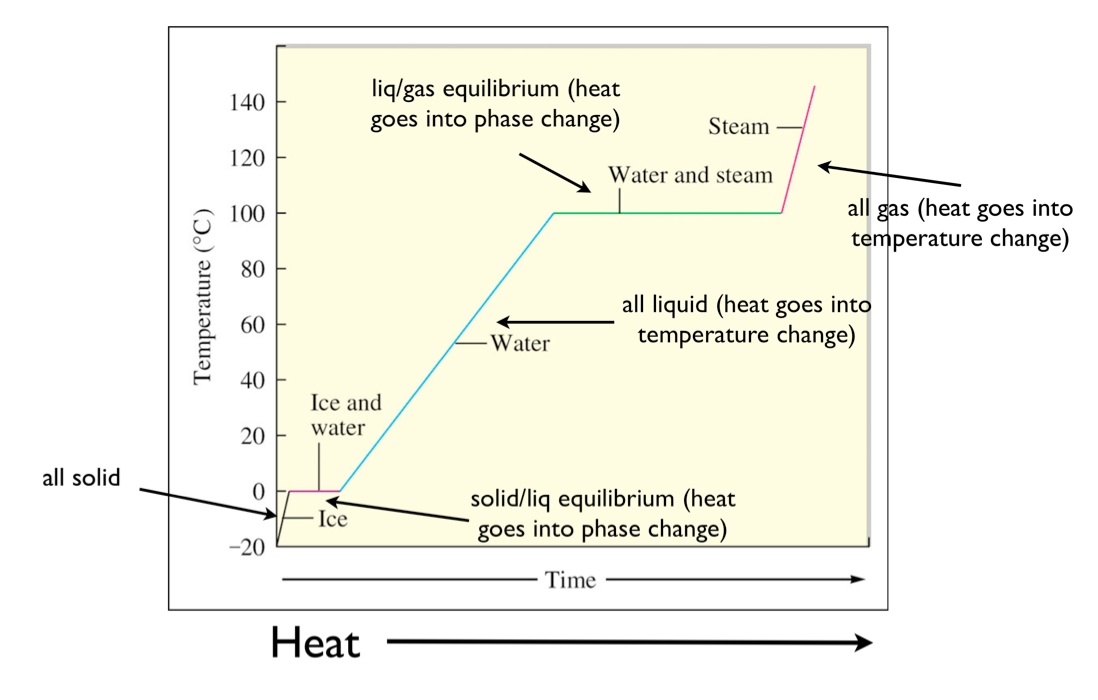
ch301.cm.utexas.eduNotice that we are on the horizontal liq/gas equilibrium line above.
Example: The latent heat of vaporization for water is 40.68 kJ/mol. How much heat would be needed to completely vaporize 36.0g of water at 100 deg C?
ANS: Since the water is already at its boiling point, there will be no heat for changing temperature (the diagonals on the diagram). All of the heat will go directly to changing the phase.
This becomes a dimensional analysis problem with the given of 36.0g
36.0gH2O1⋅1molH2O18.0gH2O⋅40.68kJ1molH2O=81.4kJ
Note:
Latent heat of vaporization is actually the total amount of enthalpy (a kind of energy/heat) necessary to accomplish a phase change for a liquid or gas at the boiling/condensation point.
Phase changes are generally considered at constant pressure, rather than constant volume. Because a kg of say, 100°C steam, occupies a much greater volume than a kg of 100°C water, a lot of work has to be done to push the environment out of the way as that water expands to become steam. (The fact that vaporization does a lot of work is why we use steam to power a large number of the turbines in the world.)
Enthalpy H includes the internal energy U that goes into the new vapor, plus the work, W=PΔV, to expand the liquid into a gas at the same pressure:
H=U+PΔV,
where P is the ambient pressure, ΔV is the change in volume, and U is the internal energy of the substance in its new phase. Latent heat of vaporization is typically much bigger than latent heat of fusion because of the much larger change in volume involved
No comments:
Post a Comment