Definition of Latent Heat
Normally when heat energy is added to or removed from an object, the temperature of the object changes; however, during phase changes, the temperature of an object stays constant. The temperature remains the same because energy is required for an object to change phases.Latent heat is the heat energy per mass unit required for a phase change to occur. If we think about substances at a molecular level, gaseous molecules have more vibration than liquid molecules. So when you add heat to a liquid, you are actually causing the molecules to vibrate. The latent heat is the energy required to change the molecular movement. Each substance has a unique latent heat value.
Formula for Latent Heat
The formula for latent heat is:Q = m * L
This equation relates the heat Q that must be added or removed for an object of mass m to change phases. The object's individual latent heat is noted by L. The unit of latent heat is J/kg.
The values of latent heat are variable depending on the nature of the phase change taking place:
- The latent heat of fusion is the change from liquid to solid.
- The latent heat of vaporization is from liquid to gas.
- The latent heat of sublimation is the change from solid to gas.
Examples of Latent Heat
In the previous example of boiling water, we know that we must continue to add heat energy to the water before all of the liquid water turns to steam. The latent heat of vaporization for water is 22.6 x 10^5 J/kg. This means that 22.6 x 10^5 J of heat energy must be added to turn one kilogram of water from liquid to gas at 100 degrees Celsius.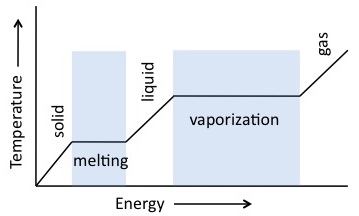 |
Thanks for the blog loaded with so many information. Stopping by your blog helped me to get what I was looking for. review
ReplyDeleteThe addition of an outside patio heater can provide benefits by, hotel, resort or restaurant owners. A stylish patio heater help keep your patio warm while expanding your parking space, that may create additional uses for your restaurant, hotel, resort or backyard patio. https://www.smartheat.org.uk/
ReplyDelete